
The entire kinetic energy energy is converted into potential energy. In case of stagnation the given mass achieves a state of constant zero or negligible velocity for practical cases. Stagnation volume is nothing but when the volume of a given gas or fluid has achieved a static condition.
The easiest to use are the DePriester charts , which cover 12 hydrocarbons (methane, ethylene, ethane, propylene, propane, isobutane, isobutylene, /i-butane, isopentane, /1-pentane, /i-hexane, and /i-heptane). For example, several major graphical i light-hydrocarbon systems. However, for mixtures of compounds of similar molecular structure and size, the K value depends mainly on temperature and pressure. 4, the i complex function of temperature, pressure, and equilibrium vapor- and hquid-phase compositions.
One cannot calculate K values until phase compositions are known, and those cannot be known until the K values are available to calculate them. Find a process flow diagram of a typical petroleum refinery that begins.pressure temperature chart, correlations for conversion between true and reid vapor, gasoline encyclopedia article citizendium, cu brookhaven national laboratory, blending vapor pressure adventures in energy, vapor pressure wikipedia, vapor pressures of alcoholgasoline blends request pdf, 5 vapor liquid equilibrium depriester chartThermodynamics 1 Simulations &leftarrow view thermodynamics 2 simulations &leftarrow view simulations organized by textbook (Elliot & Lira) Select a category All A trial-and- error procedure is required with any K-value correlation that takes into account the effect of composition. Thus by simply bringing a mixture of acetone and water to a temperature in the. The Kellogg charts, and hence the DePriester charts, are based primarily on the Benedict-Webb-Rubin equation of state , which can represent both the liquid and the vapor phases and can predict K values quite accurately when the equation constants are available for the components in question.
An alternative measure of composition is the convergence pressure of the system, which is defined as that pressure at which the Kvalues for aU the components in an isothermal mixture converge to unity. Iap tu wien, 5 vapor liquid equilibrium depriester chart statistical, measurement and prediction of reid vapor pressure of, chapter 9 distillation and vapor pressure data of diesel, pressure enthalpy charts and their use, vapor pressures of alcoholgasoline blends request pdf, propane facts amp comparison charts natural gas, how to estimate reid vaporThe Kellogg and DePriester charts and their subsequent extensions and generahzations use the molar average boiling points of the liquid and vapor phases to represent the composition effect. SI versions of these charts have been developed by Dadyburjor. These nomographs are shown in Fig.

Take the equilibrium data from the Depriester charts given in Chapter 8. Calculate the flow and composition of the liquid and vapour phases. A feed to a column has the composition given in the table below, and is at a pressure of 14 bar and a temperature of 60☌. The DePriester charts check this quite well (see Figures 8-4A and B, and Figure 8-3D). For n-pentane at convergence pressure of 3,000 psia (nearest chart) the K-value reads 0.19.
The bubble point of the feed at 400 psia (27.6 bar) and at the feed composition, calculated using ASPEN , is 86.5 ☏ (130 ☌). The relative volatility depends on temperature and pressure. To obtain the composition of the top and bottom products, first calculate the relative volatility of each component using the conditions of the feed as a first guess.
Water Depriester Chart Series Of Light
The DePriester Charts for the homologous series of light hydrocarbons can serve as a basis with approximate validity for simple calculations of VLE situations. 7 (1953), ail rights reserved.Originally there were charts prepared to make use of these definitions. Used by permission DePnester, C.L., The American Institute of Chemical Engineers, Chemical Eng. DePriester Charts K-Values of light hydrocarbon systems, generalized oorreiations, low-temperature range. Next, calculate the relative volatility of the feed stream, defined by Equation 6.27.18, for each component relative to the heavy key component. Bubble and dew points could also be calculated using K-values from the DePriester charts and by using the calculation procedures given in Chapter 3.
Units) at low tenperatures.Figure 2-12. Modified DePriester chart (in S.I. The DePriester charts have been fit to the following equation (McWilliams, 1973). Approximate values can be determined from the monographs prepared by DePriester (1953). This allows m-values for light hydrocarbons to be calculated and correlated as functions of T and P. However, in the case of mixtures of light hydrocarbons, we may assume as a reasonable approximation that both the liquid and the vapor phases are ideal.
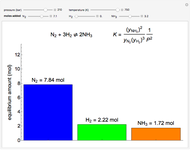
(2=30) is used for the K values. Essentially the same answer is obtained if Eq. They aren t perfect, because V/F wasn t exact. Calculate V from V/F and L from overall mass balance, Eq. Once the correct V/F has been found, calculate X from Eq. (2=46), to converge on the correct V/F value.


 0 kommentar(er)
0 kommentar(er)
